The Walker Lab
Welcome to Our Lab
We combine multiple scientific disciplines to define the host-pathogen interactions that determine the onset, course, and outcome of chronic infections. Using medical device infections as a model for chronic infections, we are currently pursuing the following questions:
How do medical devices render people susceptible to infection?
Millions of medical devices are placed every year, and their use is expected to increase due to their efficacy at improving the length and quality of life. However, infection of medical devices is a common, dreaded complication. Our work indicates the device itself induces inflammation, which may prevent the host from mounting an effective response against pathogens, allowing them to cause disease. To define the host-pathogen-device interactions, we combine the use of model systems and patient samples to understand the inflammatory response to devices with and without infection to identify biomarkers that predict infection risk.
2. What microbial virulence mechanisms increase disease severity and complications associated with medical device infections?
A broad range of microbes form chronic, recalcitrant medical device infections. Our group studies the different virulence mechanisms these microbes use to initiate infection, form recalcitrant communities, and progress to severe disease, including bacteremia and sepsis. Our work demonstrates that microbes, including Staphylococci, Candida albicans, and Pseudomonas aeruginosa, attach to various host proteins that coat device surfaces and form recalcitrant biofilms that resist antibiotics. Additionally, these microbes use sensor regulatory systems to coordinate the response of virulence factors, including adhesins, enzymes, and proteases, to initiate and progress to severe infection. To define the microbial mechanisms that facilitate infection, we combine the use of microbial genetics and molecular microbiology to inform the development of novel antibiotic-sparing prevention and treatment strategies.
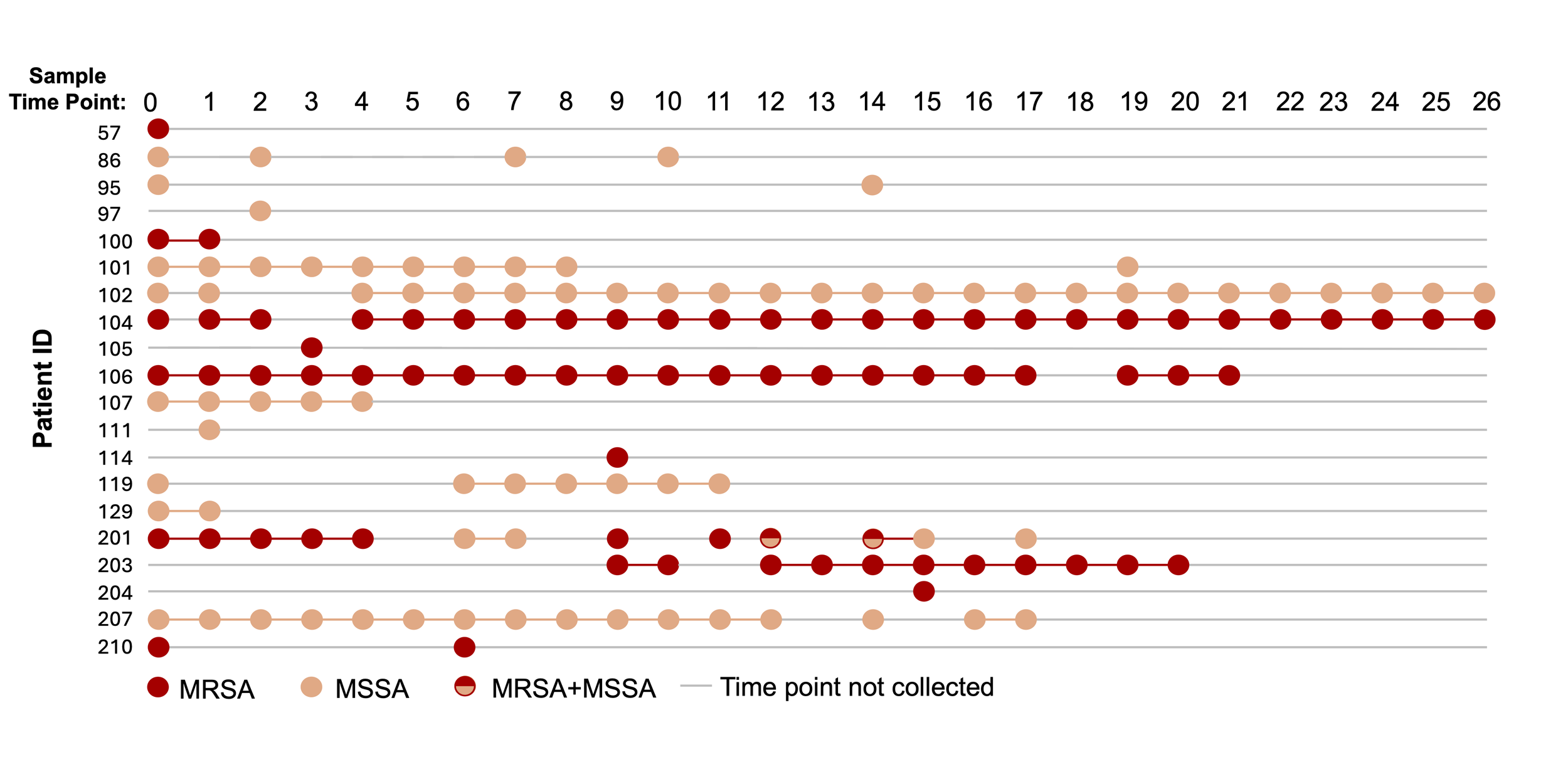
3. How do genomic adaptions during long-term colonization impact persistence and pathogenic potential?
Many medical devices become asymptomatically colonized with pathogens. Chronic colonization increases the risk of developing symptomatic infection. Our genomic studies with Staphylococcus aureus and Klebsiella pneumonia and other microbes, indicate that these microbes acquire genomic changes that promote tissue tropism and increase pathogenic potential (figure generated by rotation student Alexandra Serrato). We use a combination of epidemiology, genomics and bioinformatics, patient samples, and model systems to understand how bacteria evolve to persist asymptomatically long-term and adapt to become more pathogenic to help to guide the development of more effective prevention and treatment strategies.
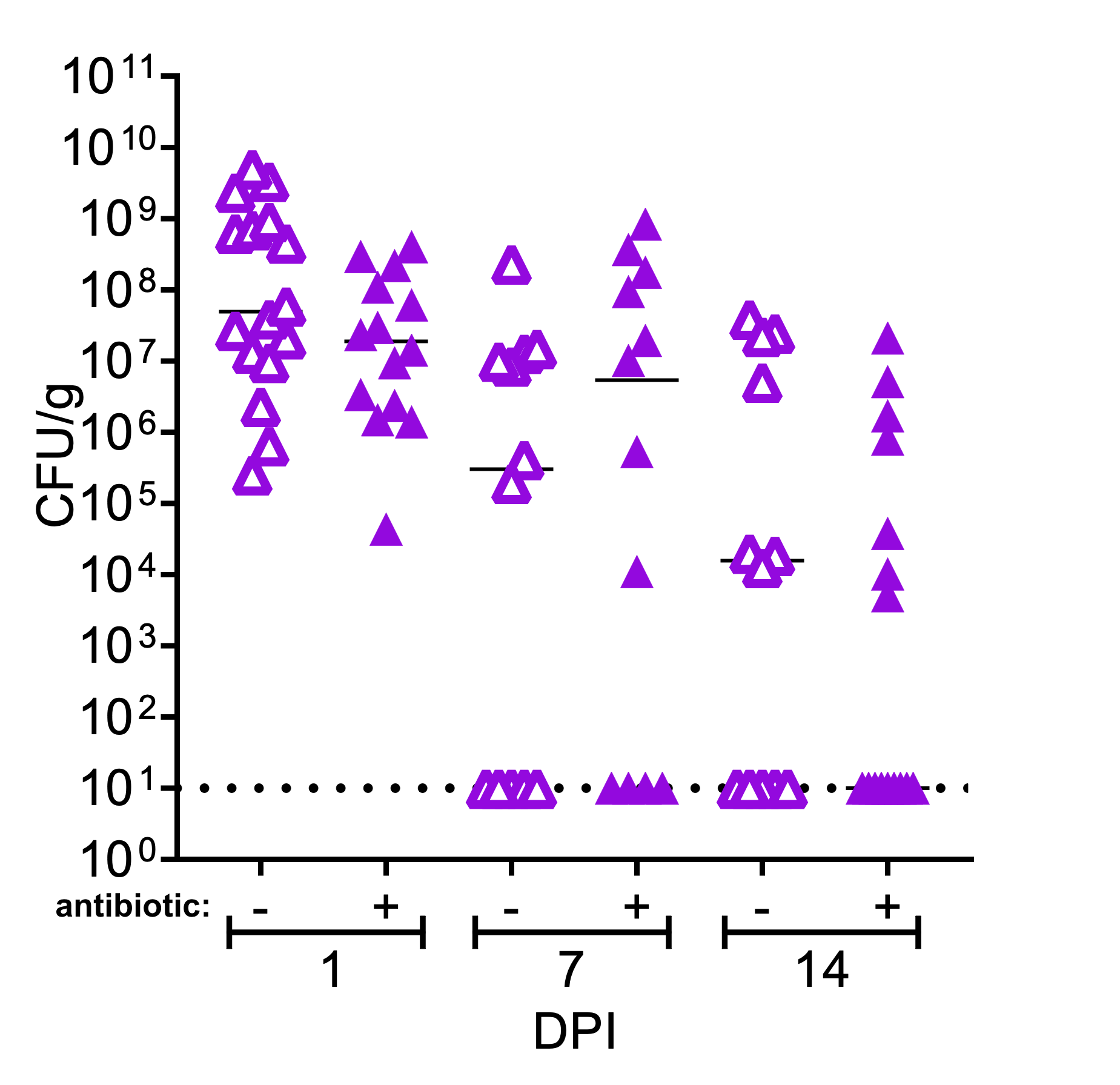
4. What therapeutic strategies are effective against medical device infections?
Medical device infections resist antibiotic therapy, and the current gold standard of treatment relies on device removal. Our group recently discovered that antibiotic pocket irrigants used during breast reconstruction post-mastectomy can enhance biofilm formation among clinically derived infection isolates and do not prevent infection in an animal model. We collaborate closely with physicians and use a combination of model systems patient and samples to understand how bacteria establish recalcitrant infections and translate these discoveries to guide prevention and/or treatment strategies.