Research

Urinary Catheter Infections
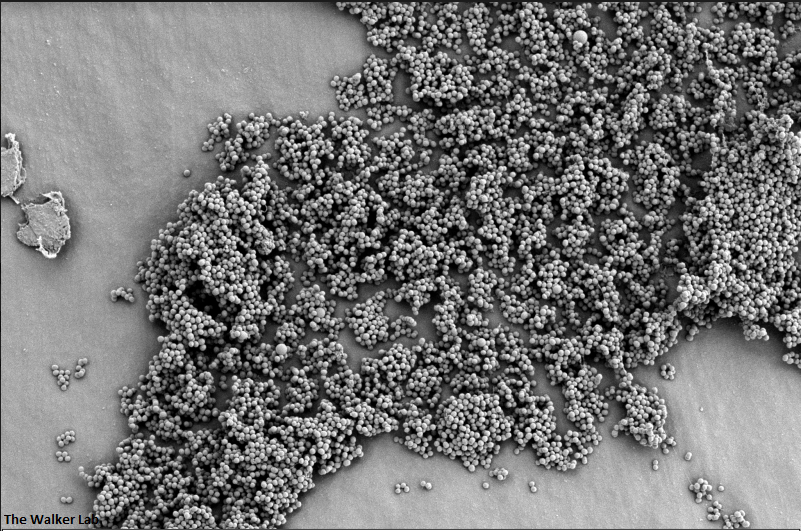
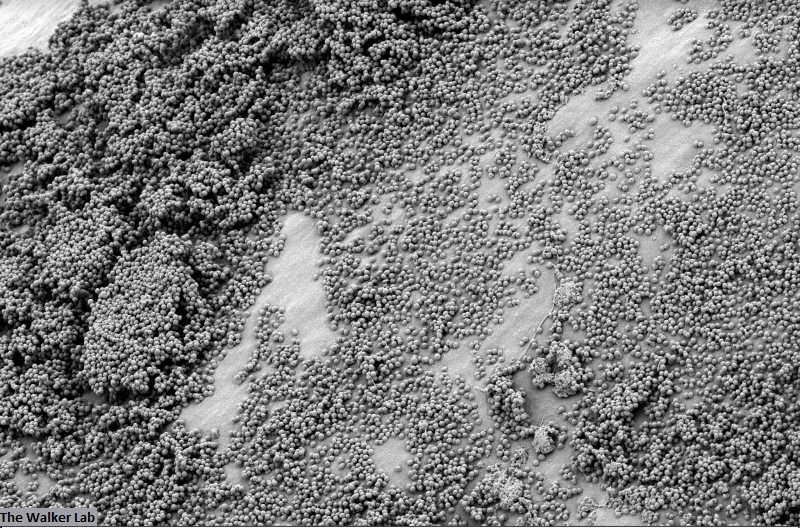
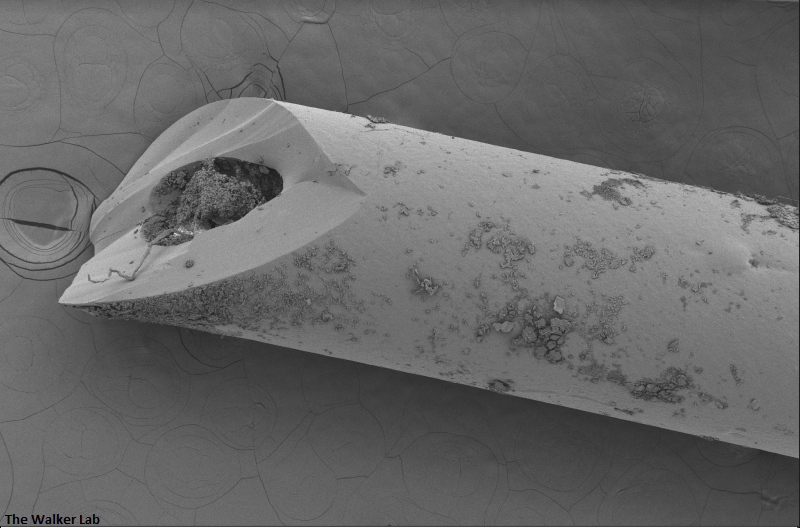
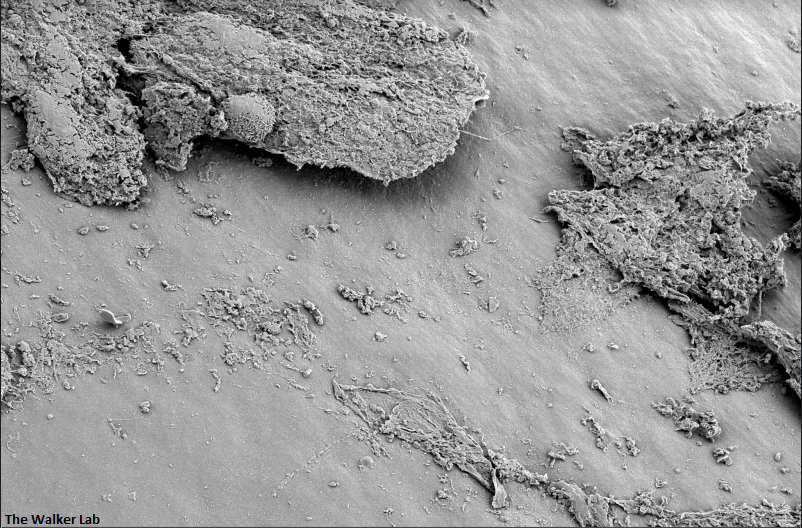
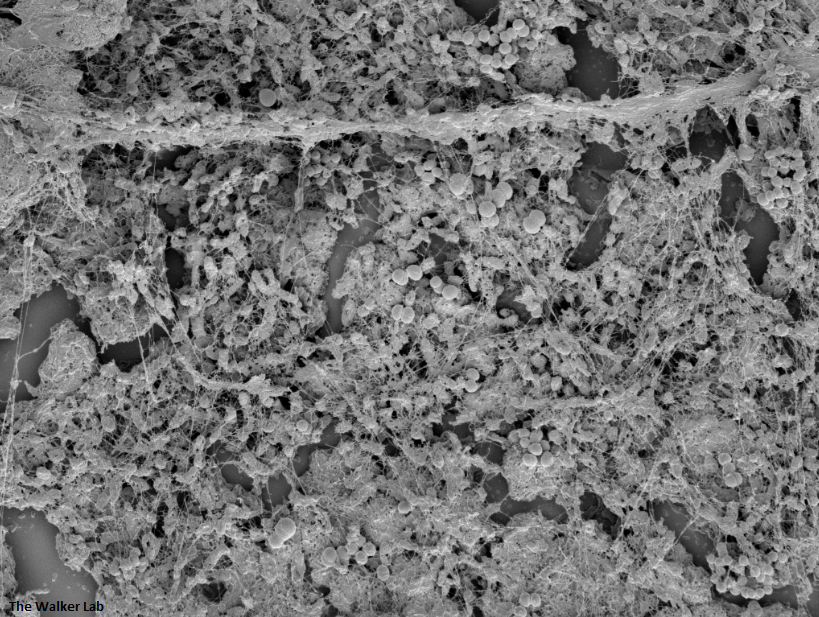
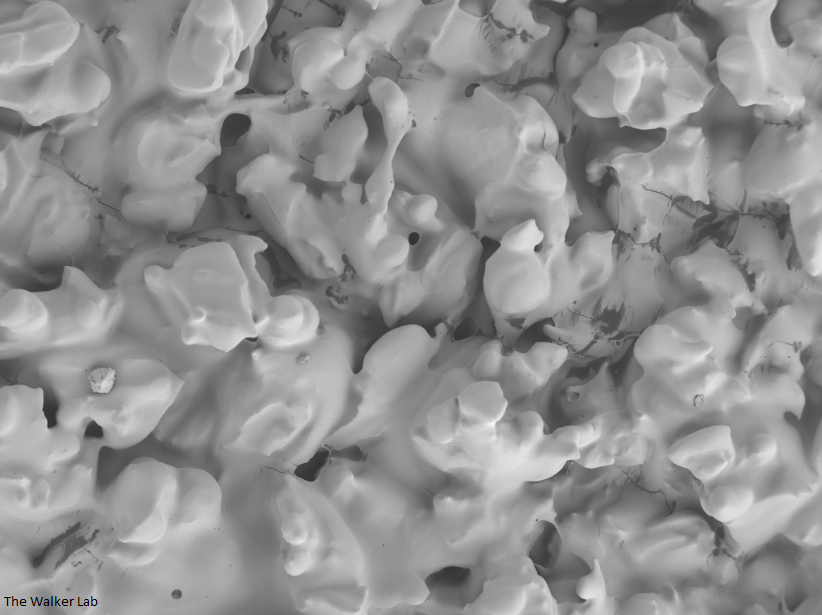
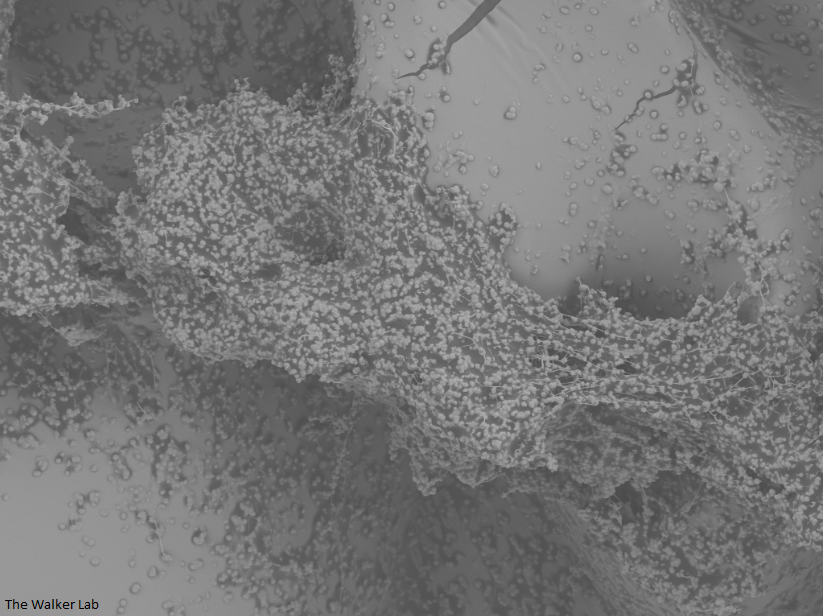
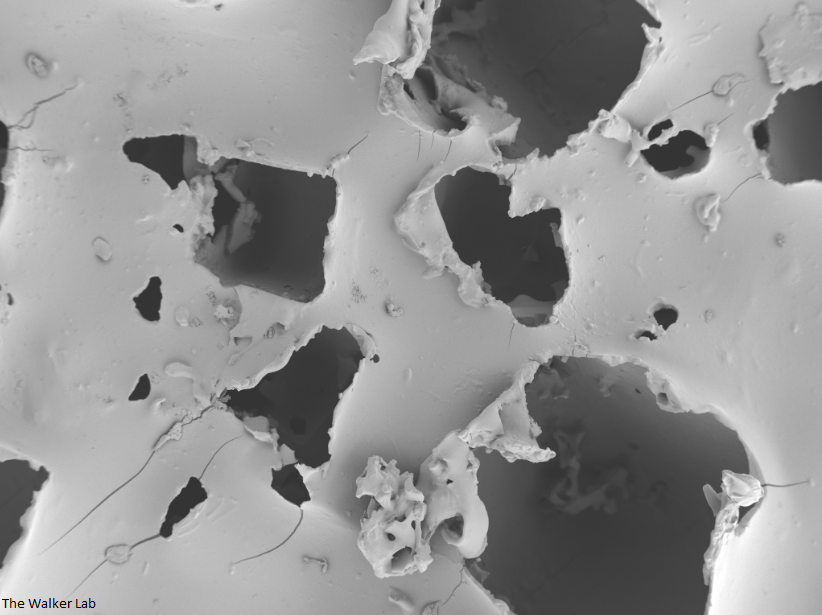
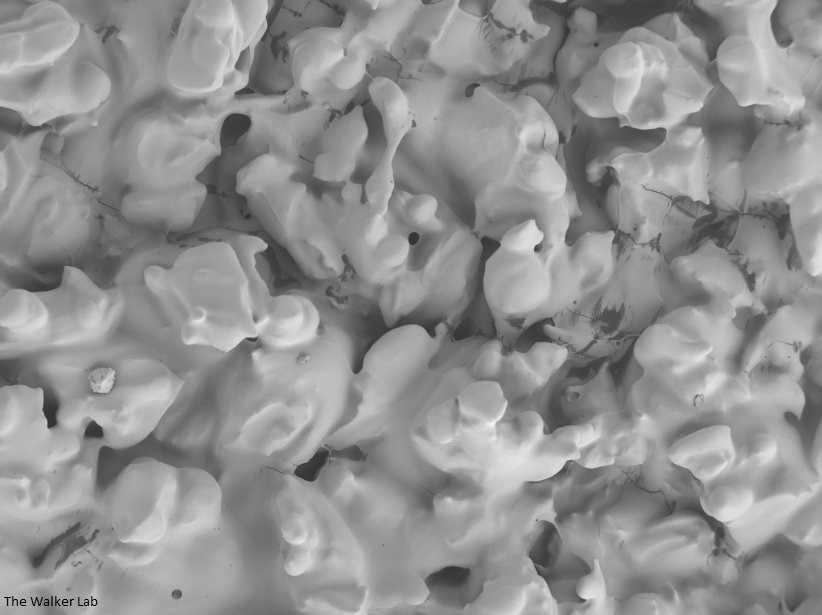
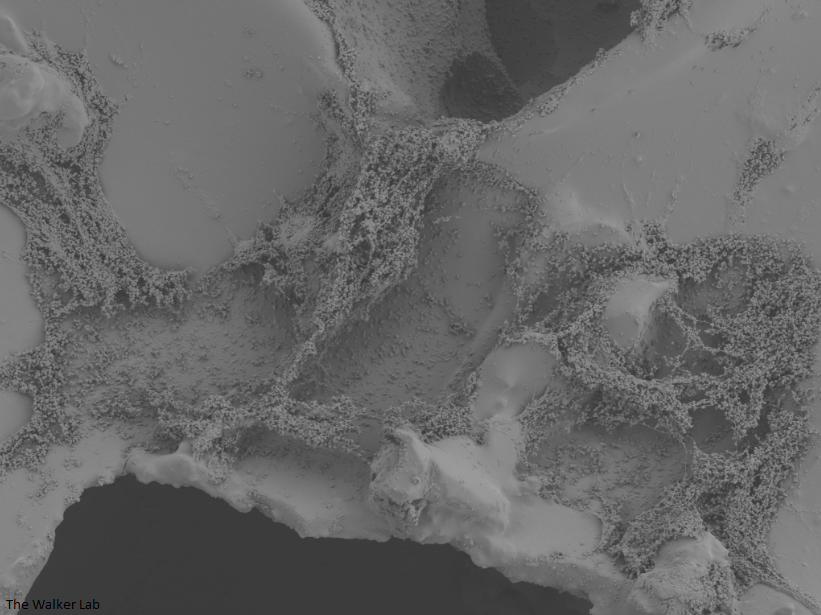
SEM Images of Mouse Catheters
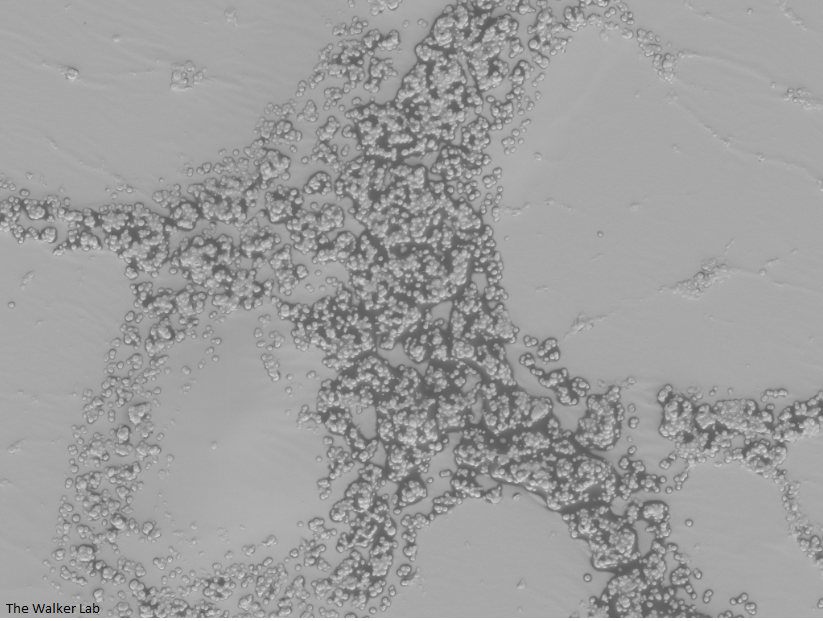
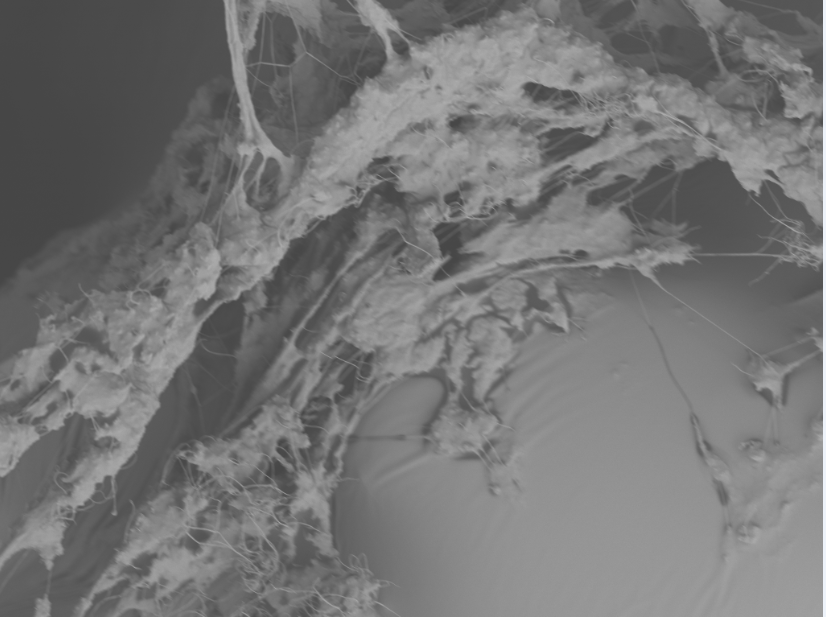
Silicone urinary catheters have smooth surfaces, while latex urinary catheter surfaces are rough. Regardless of the catheter material, deposition of host inflammatory factors alters the surface and facilitates infection by atypical pathogens.
Latex Catheter Surface
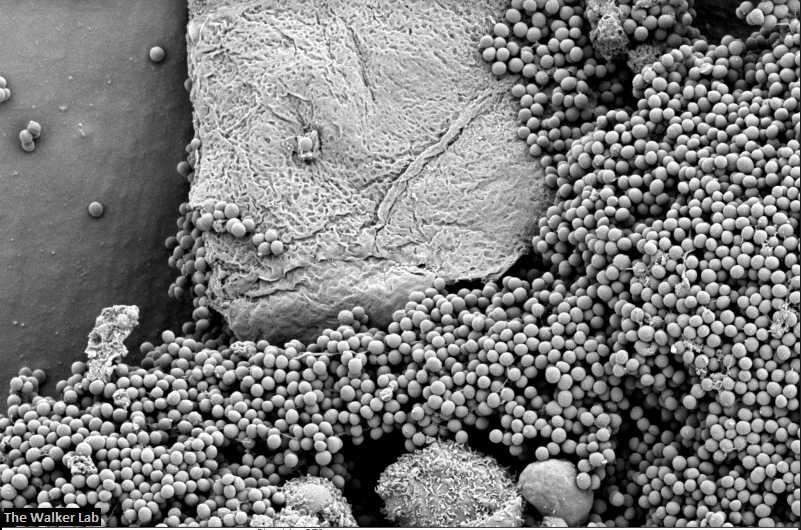
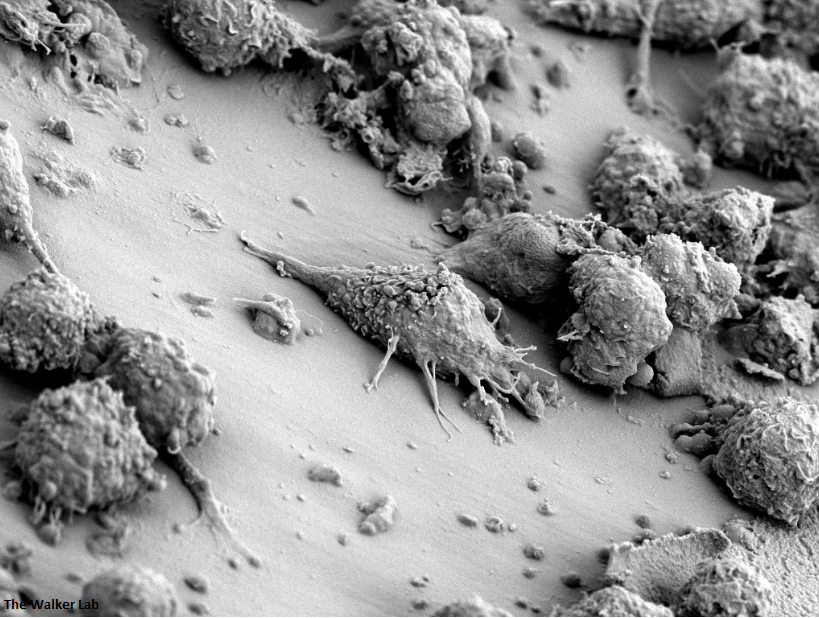
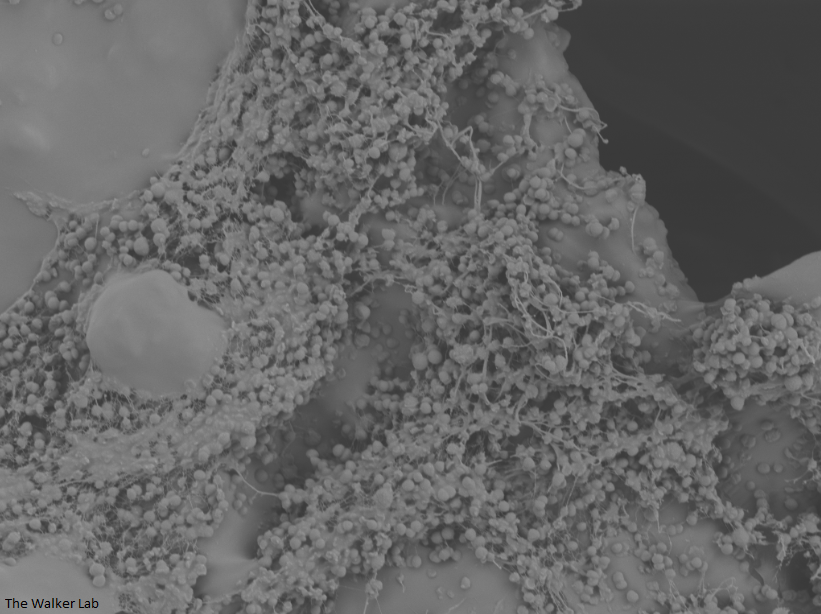
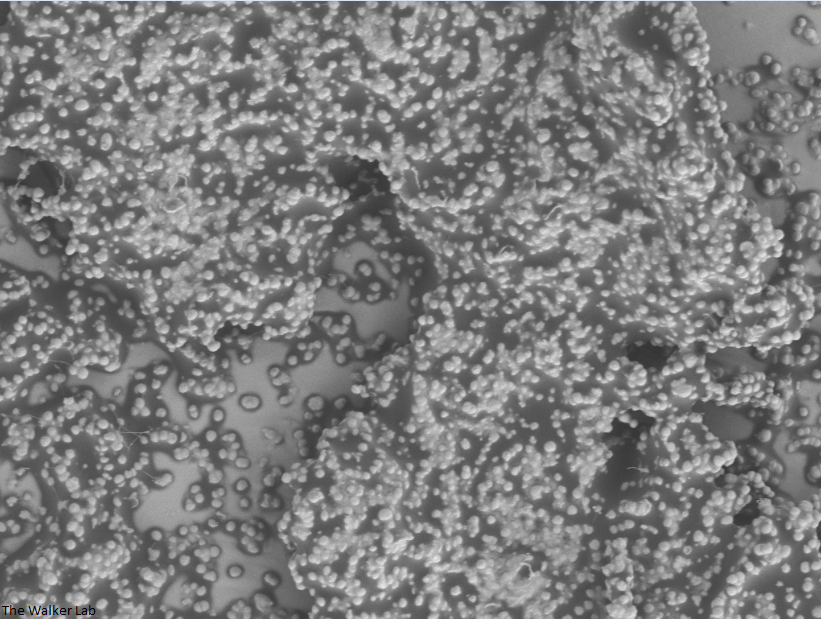
Host Immune Cells and Glycoproteins
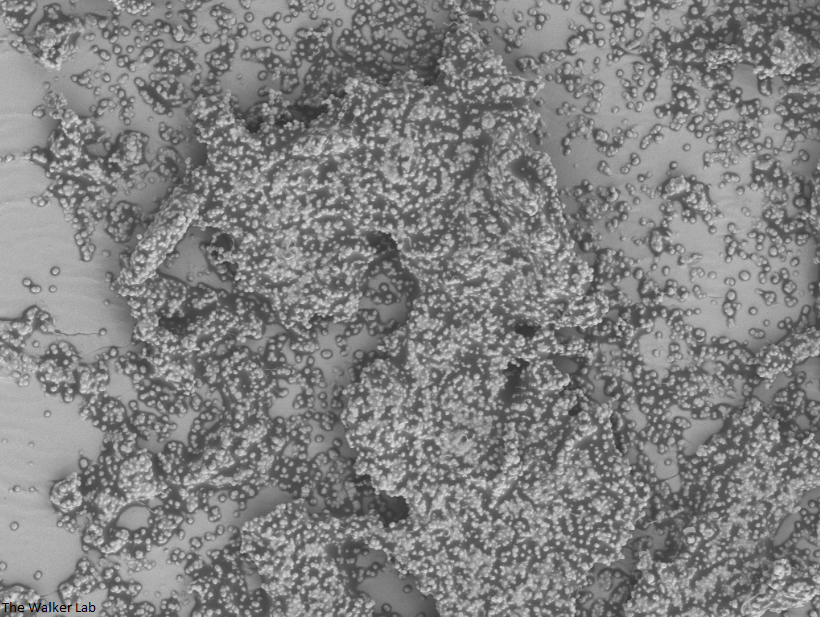
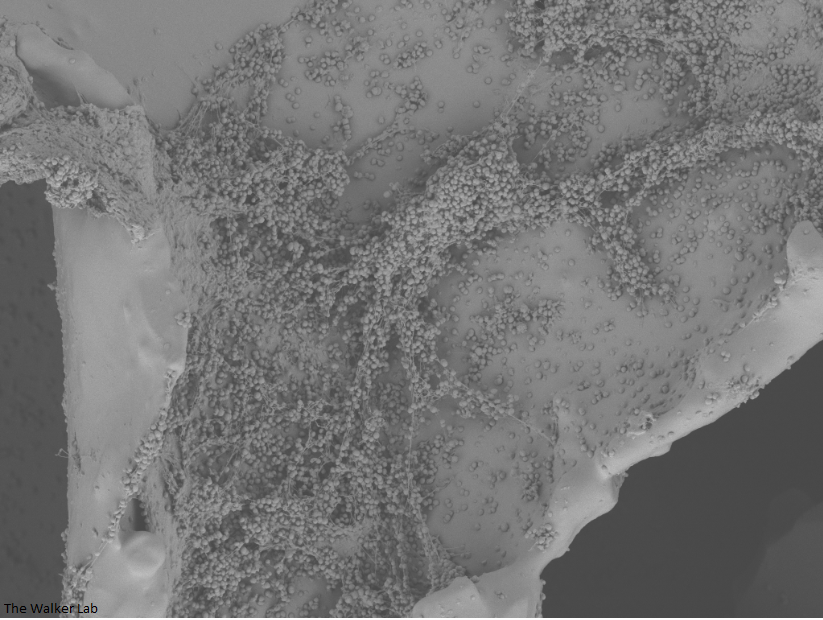
Bladder Epithelial Cells
Urinary catheters render people susceptible to atypical uropathogens. We use the atypical uropathogen, methicillin-resistant Staphylococcus aureus (MRSA), to study the catheter-host-pathogen interactions that facilitate disease.
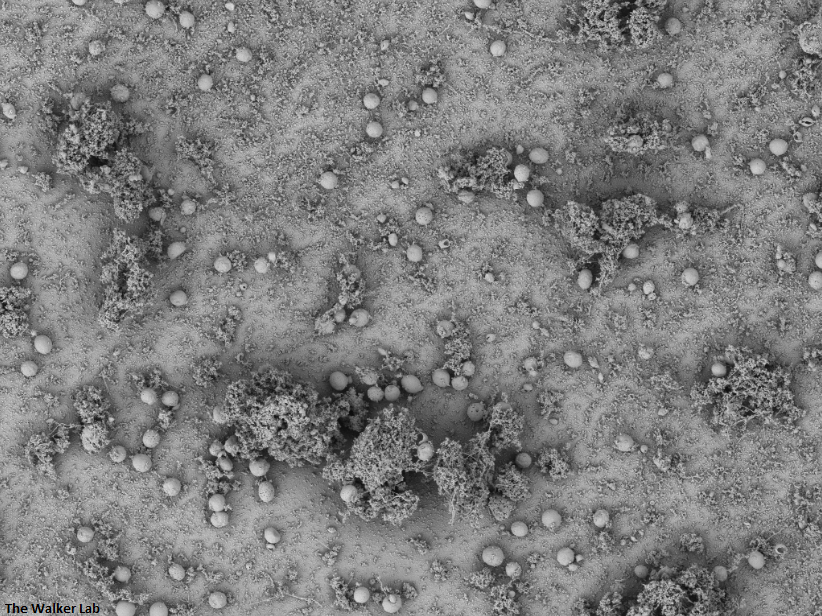
Host Immune Cells and S. aureus
Host Immune Cells and S. aureus
Host Immune Cells, S. aureus, Pseudomonas
The host inflammation response to the catheter recruits immune cells and wound healing proteins which are deposited on the catheter surface. Atypical pathogens can use these host factors to adhere to the catheter's large surface and create biofilm. Colonization of catheters occurs quicker than expected and develops even in the presence of prophylactic antibiotic therapy. MRSA is just one of the uropathogens that can attach to the altered urinary catheter surface. One host factor deposited on the catheter surface is fibrinogen. We recently discovered that MRSA binds fibrinogen to attach to the catheter surface and form biofilm, which increases antibiotic recalcitrance. Our future studies aim to develop new non-antibiotic strategies that interfere with those interactions to prevent or treat CAUTIs.

Breast Implant Infections
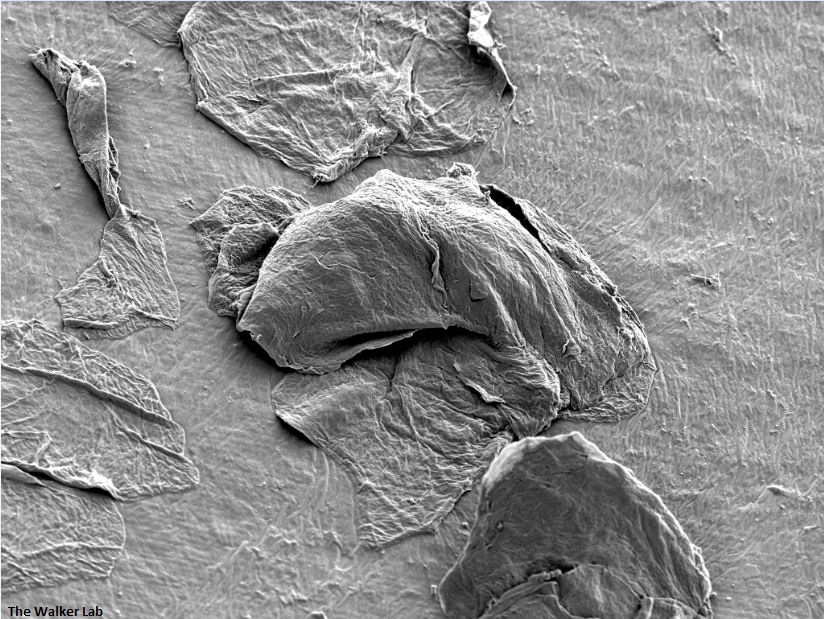
SEM Breast Implant Images
Breast implant surfaces are made of silicone. However, surface texturing is used to increase tissue adhesion and prevent movement following placement.
Texture Breast Surface
Smooth Breast Surface
Microtexture Breast Implant
The deposition of wound-healing proteins on the implant surface facilitates staphylococcal infection.
Incorporation of host collagen into S. epidermidis biofilm
Incorporation of host fibrinogen into S. epidermidis biofilm
Incorporation of host collagen into S. epidermidis biofilm
Similarly to the urinary catheters, staphylococci, including S. aureus and S. epidermidis, exploit the deposition of wound-healing glycoproteins to bind to the implant surface. Textured breast implant surfaces have increased host glycoprotein deposition and therefore higher bacterial colonization. Staphylococci and Pseudomonas aeruginosa are the primary Gram-positive and Gram-negative cause of breast implant-associated infections. We have developed a mouse model to study the implant-host-pathogen interactions that facilitate infection. Our future studies aim to translate the results from these studies into better surveillance, prevention, and treatment strategies.
Antibiotic Resistance
Bacteria exhibit both genotypic and phenotypic tolerance to antimicrobials. Genotypic tolerance leads to genetics changes that leave the bacteria intrinsically resistant to commonly used antibiotics, ultimately limiting treatment options. Phenotype tolerance typically occurs when susceptible planktonic bacteria form communities, such as biofilms, that provide a physical barrier to antibiotics, limiting antimicrobial effectiveness. We seek to investigate the genotypic and phenotypic bacterial mechanisms that facilitate recalcitrance to antibiotic treatment. Our future studies aim to develop new non-antibiotic strategies that prevent or disrupt those communities to effectively treat medical device-associated infections.